The Unstoppable FemTech Movement towards Optimum Women's Health
Nov 24, 2022
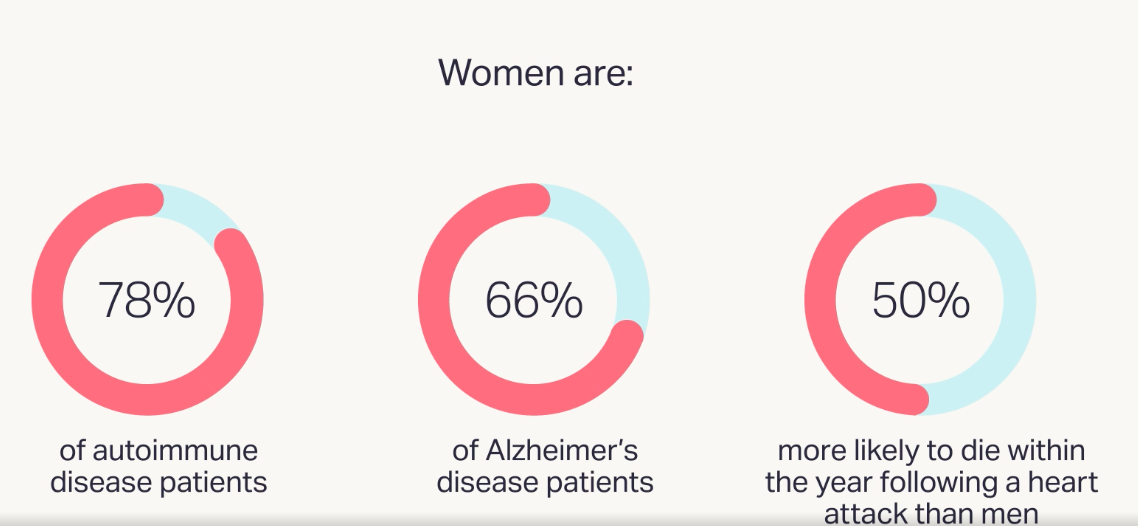
Recent statistics about women's health summarized nicely on WHAM website are enlightening.
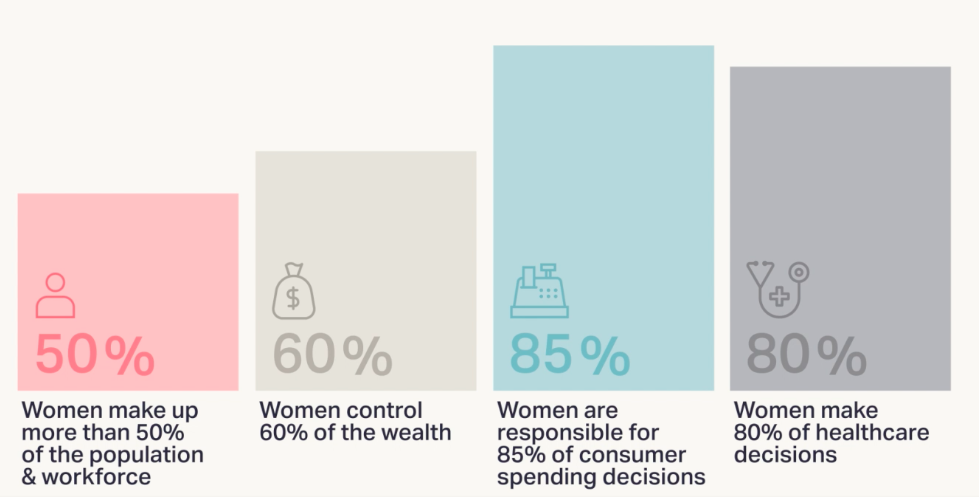
However, women’s healthcare based on substantial clinical trials with female participants is severely lacking.
Femtech is a term that refers to diagnostic tools, products, services, wearables, and software that use technology to address women's health issues. It can include menstrual health, reproductive health, sexual health, maternal health, and menopause.
FemTech focuses on providing affordable, self-care women's health solutions concurrently with medical care. Most companies base their products on science and clinical trials to help women better manage their health throughout their lifetime.
Society has essentially silenced women from talking about many health issues, calling them taboo or inappropriate. Our periods, urinary problems, postpartum prolapses, fertility challenges, perimenopausal hot flashes, pain with sex, and so much more are off the table. All of these things are expected to be hidden.
The recent increase in FemTech and growing funding for these companies that conduct clinical trials with female participants are shifting the narrative.
Clinical trials play a HUGE role in health care. These studies determine the quality of medications, best dosages, and side effects. The surgical techniques that are most safe and effective, and how behavioral changes such as regular exercise and mindfulness practice improves health.
Medical intervention doesn't just appear out of nowhere; it takes decades of research, clinical trials, and investment.

Before 1993, all clinical trials were conducted on males. Women were forbidden to be participants in studies. Men were the medical norm until Congress passed the NIH Revitalization Act, which mandated the inclusion of women and minorities in clinical trials.
Women were historically excluded from clinical trials for a few main reasons such as:
- Gender bias; the assumption that there were no significant sex differences regarding medication response, and therefore no need to study women separately.
- Concern over having to adjust for women's fluctuating hormone levels.
- Concern over reproductive effects.
Women were, in essence, considered small men.
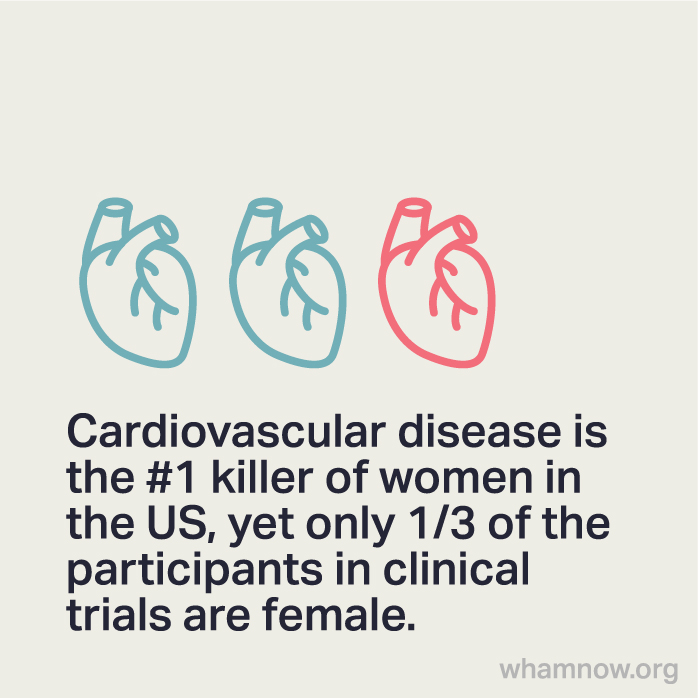
One big issue for not including women is that some diagnoses, like heart disease, present differently in men vs. women. How cholesterol plaque in the arteries looks in men is different than how it shows up in women. As a result, heart disease is underdiagnosed in women because they don't have the same presentation as men.

A second big issue with omitting women from clinical trials is prescription dosages. Men and women have different body fat compositions, and women typically have smaller frames than men. Without studies, we don't know whether there should be a difference in drug dosages.
Funding is a big issue for women's health.
In fact, in nearly three-quarters of cases where a disease primarily affects one gender, the so-called "men's diseases" are overfunded. In contrast, "women's diseases," such as autoimmune diseases, are dramatically underfunded.
It's easy to sit back and think: well, this has improved from 29 years ago, and we've come a long way, haven't we? While ANY progress is good, a 2019 study found that women are still underrepresented in clinical trials.

The burgeoning growth of FemTech is helping to move the dial.
The founders starting women's health companies (over 90% women) are STEM engineers, medical practitioners, business insight people, and women who are tired of the status quo and now have the skill sets to do something about it.
More advocacy groups are advocating for women's equality in clinical research. Shikha Jain, MD, FACP, co-founded the Women in Medicine Summit just three years ago. The summit is a global conference focused on empowering women and men to close the gender gap in medical research on a personal and national level.
Another group called Women's Health Access Matters (WHAM) is a nonprofit advocacy organization working to increase awareness of and funding for women's health issues.
While we have a long way to go, consider supporting FemTech founders, investing in their company, trying their products, and perhaps participating in a clinical trial. Many studies look for healthy people, so you don't have to be sick to engage in a clinical trial.
To find opportunities, check ClinicalTrials.gov or check with your local health department. At present, the site has 433,579 research studies to explore in all 50 states and 221 countries.
Your support of FemTech and participation in clinical trials will further health insights for yourself and other women. Check out my company PelvicSense and tell your friends about this online solution for pelvic floor dysfunction/pain.
Image credits: WHAM

